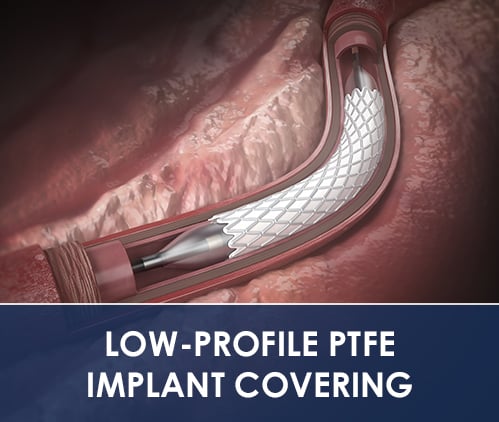
PTFE Implant Covering
Aran Biomedical offers PTFE stent encapsulation services for balloon and self-expanding stents. The company has significant expertise covering even the most challenging stent geometries and ensures minimum impact to sheath size when moving from a bare metal design. During the development phase, material encapsulation can be optimised to suit individual stent designs and the indication for use. Multi-axial laser processing ensures an optimum finish for the covered stents and the scope to integrate features into the stent wall.
PTFE (PolyTetraFluoroEthylene)
PTFE is a well-established biomaterial with a long clinical history of implantation. It has been approved by regulatory bodies for several decades and is used extensively in a range of vascular indications.
The unique physical & mechanical characteristics of PTFE enable it’s use as a stand-alone, surgically implanted material, or for direct attachment to an implantable frame for transcatheter delivery.

PTFE Implant Optimisation
- Seamless attachment to implantable frames
- Suitable for balloon & self-expanding devices
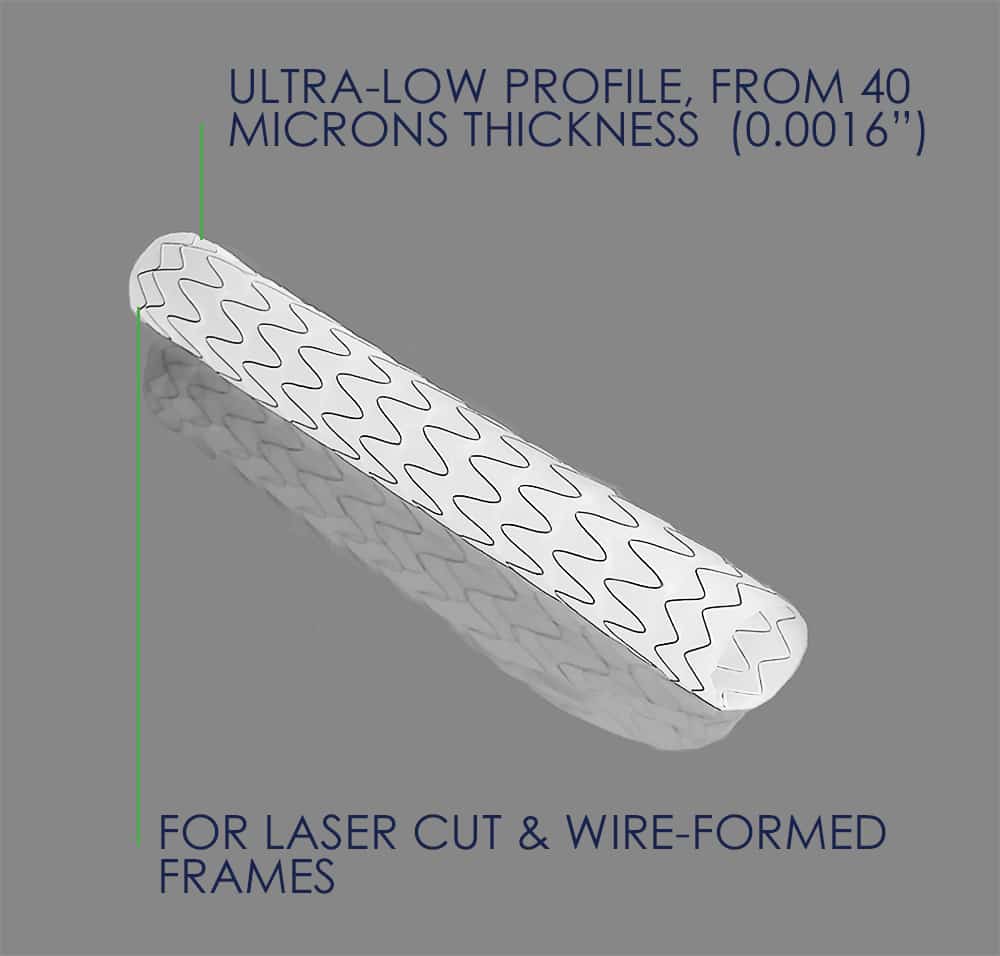
- Thicknesses from as low as 40 microns (0.0016”)
- Tailored inter-nodal distance for tissue ingrowth
- Optimise covering permeability to prevent leakage
- Mechanical properties refined and characterised
- Cover complex geometries including contours & flares
- Aran Biomedical can also offer seamless bifurcated & trifurcated designs
Why Cover with Aran Biomedical?
Aran Biomedical specialises in the development and manufacturing of implantable devices and has been working with ePTFE for over a decade. The company offers bespoke design and development solutions with PTFE, for use in either surgically or transcatheter implanted devices. Aran Biomedical biomaterials expertise and materials processing equipment can precisely tailor PTFE implants to meet specified performance requirements. Operating under a robust Quality Management System, the company is ISO 13485 certified and has been audited by the FDA and other regulatory bodies.
Development Process
– Determine feasibility through proof-of-concept prototyping
– Optimise the functional performance of your cover/ implant
– Reduce your next generation device’s covering profile
– Cover complex implantable devices and assemblies
– Transfer your existing commercial product manufacturing
– Accelerate your product development timeline
– Bulk non-Sterile supply or as finished, packaged devices

