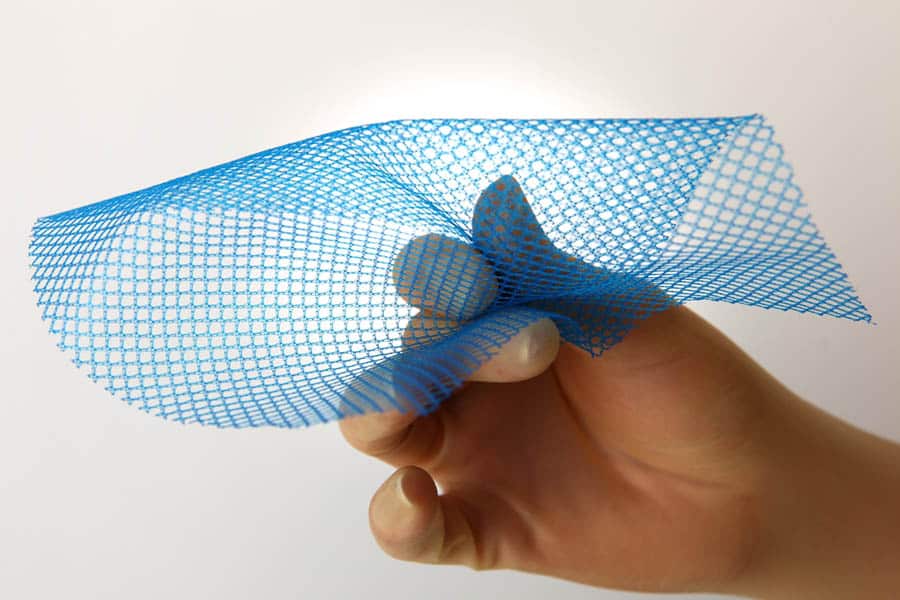
Jun-2011 - VitaMesh Blue Light-Weight Surgical Mesh Obtained CE-mark and FDA Clearance
Proxy Biomedical’s VitaMesh BlueTM Light-Weight Condensed PP Surgical Mesh was cleared for sale in US and Europe in 2011

Proxy Biomedical’s VitaMesh BlueTM Light-Weight Condensed PP Surgical Mesh was cleared for sale in US and Europe in 2011